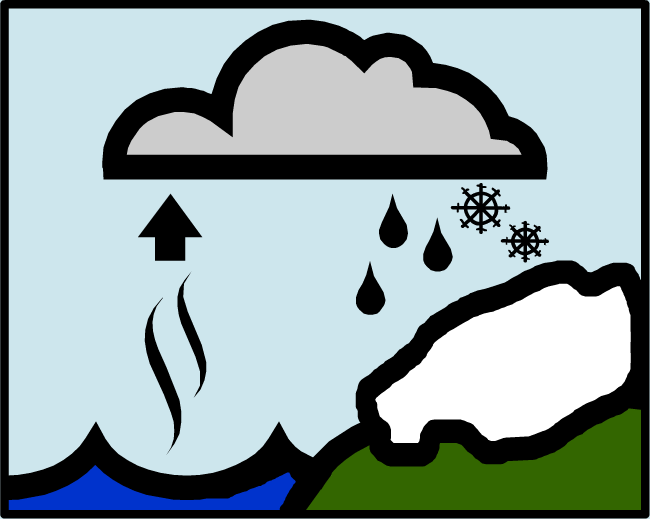
 Stable isotope ratios are highest in ocean water, lowest in water vapor and clouds, and intermediate in precipitation. This is due to isotopic fractionation that occurs during the processes of evaporation and condensation.
Stable isotope ratios are highest in ocean water, lowest in water vapor and clouds, and intermediate in precipitation. This is due to isotopic fractionation that occurs during the processes of evaporation and condensation.
The majority of water (H2O) molecules are composed of only 16O and 1H isotopes, but a small percentage contain the heavier stable isotopes 18O and D (2H, or deuterium). When water in oceans and lakes evaporates into the atmosphere, water molecules composed of lighter isotopes evaporate more readily than those with heavier isotopes. As a result, water vapor in the atmosphere is isotopically lighter than ocean water.
When water vapor then condenses to form precipitation, heavier isotopes condense more readily than lighter ones, causing the 18O/16O and D/H ratios of precipitation to be less than that of ocean water, but greater than that of the water vapor. This process is often referred to as isotopic fractionation.
Next Page